Introduction
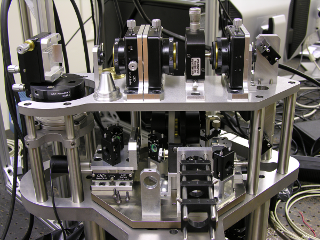
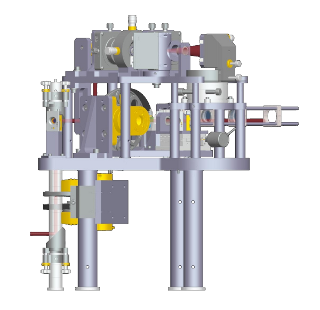
Distant Focus was awarded an SBIR phase I contract in 2005 and a Phase II contract in 2006 to design and construct a dual function optical coherence microscope (OCM) and multi-photon fluorescence microscope (MPM). This laser scanning microscope is was designed to assist NIST (National Institute of Standards and Technology) personnel research tissue engineered medical products. Distant Focus has teamed with Professor Stephen Boppart of the University of Illinois to transfer technology to near-commercial realization while assuring that the instrument meets stringent performance objectives.
Description
Optical coherence microscopy (OCM) extends the capabilities of OCT and confocal
microscopy by combining high sensitivity and coherence gated detection with
confocal optical sectioning. The result is an improvement in the rejection of
undesirable light scattered from outside the imaging region thereby leading to
a dramatic contrast enhancement in imaging structural elements. Multi-photon
fluorescence microscopy (MPM) has become a well established optical imaging
technique for acquiring functional information. This microscope simultaneously
acquires OCM and MPM information through layered, high resolution 2D scans
of the sample.